
GI Genius™
Real time AI, redefining colonoscopy
GI Genius™ is the first deep learning–based, real-time AI system designed to support gastroenterologists during colonoscopy.
Medtronic is the sole distributor, marketer and owner of GI Genius trademarks.

Greater confidence. Greater care.
Powered by Artificial Intelligence, the GI Genius™ intelligent endoscopy module offers a transformative solution to address challenges in colorectal screening. Integrating seamlessly with existing endoscopy processes, it harnesses deep learning algorithms to assist in clinical decision-making in real time, using visual markers to alert physicians to the presence of lesions.
Clinical evidence shows that GI Genius™ significantly improves diagnostic performance compared to standard high-definition white-light colonoscopy giving clinicians greater accuracy and patients greater confidence.
Seamless integration. Effortless flow.
Designed for seamless integration, GI Genius™ works with all major endoscopic processors fitting effortlessly into existing workflows without the need for major equipment upgrades.
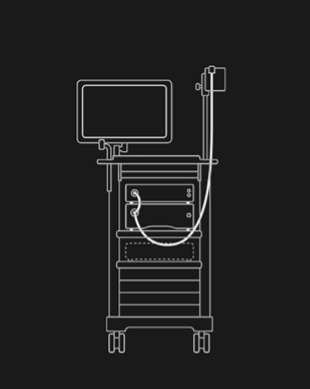
Your existing endoscopy tower and high-definition endoscope is all you need to integrate with the GI Genius™ intelligent endoscopy module.
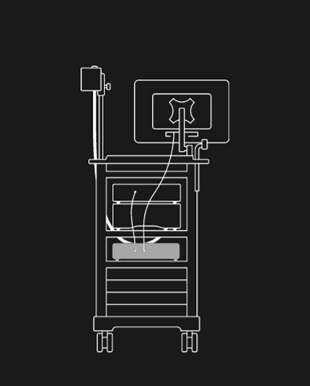
GI Genius™ intelligent endoscopy module can be easily integrated with existing major brands and endoscopic processors.
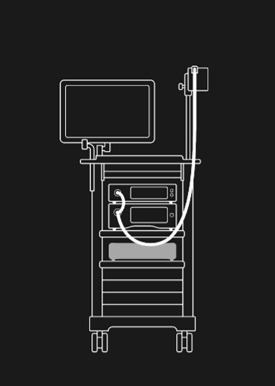
GI Genius™ intelligent endoscopy module simply connects to the existing endoscope, video processor, and display monitor.
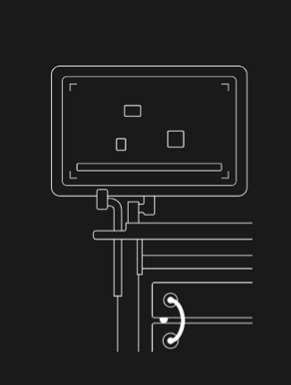
Turn on GI Genius™ intelligent endoscopy module and immediately experience the benefits of AI, without changing any part of your procedure.
Proven to enhance detection
Clinical evidence demonstrates that GI Genius™ significantly improves diagnostic performance compared to standard high-definition white-light colonoscopy.
GI Genius™ in numbers
Absolute increase in adenoma detection rate 8.2%
Adenoma detection rate (ADR)
56.6%
GI Genius™ polyp detection demonstrates consistent improvement in adenoma detection across multiple large-scale studies. The largest real-world, randomized controlled, multi center COLO-DETECT trial2, showed an 8.2% absolute increase in adenoma detection rate (56.6% vs 48.8%) and a 38% relative increase in sessile serrated lesions detected per procedure (10.9% vs 7.9%).
≈50%
GI Genius™ polyp detection halves adenoma miss rate.1
15.5%
with GI Genius™
VS
32.4%
in conventional high-definition colonoscopy.
Unnecessary polypectomy reduced by half
Genius™ polyp characterization cuts the burden of unnecessary polypectomy by half3. Landmark RCT published in The Lancet Gastroenterology & Hepatology in 2025 showed that GI Genius™-supported optical diagnosis makes leave-in-situ strategy oncologically safe for clinical practice.
50%
Reduction in unnecessary polypectomy
Non-expert endoscopists brought to level of expert endoscopists with GI Genius™ polyp characterization.4
97.6%
Cost saving strategies with negative predictive value of 97.6% for diminutive rectosigmoid lesions.5
53%
increase in the likelihood of detecting polyps in the distal colon.
46%
increase in the number of adenomas detected per colonoscopy.6
M. B. Wallace et al., ‘Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia’,
Gastroenterology, pp. S0016-5085(22)00238–4, Mar. 2022,
doi: 10.1053/j.gastro.2022.03.007.
A. Seager et al., ‘Polyp detection with colonoscopy assisted by the GI Genius artificial intelligence endoscopy module compared with standard colonoscopy in routine colonoscopy practice
(COLO-DETECT): a multicentre, open-label, parallel-arm, pragmatic randomised controlled trial’,
Lancet Gastroenterol. Hepatol., vol. 9, no. 10, pp. 911–923, Oct. 2024, doi: 10.1016/S2468-1253(24)00161-4.
G. Antonelli, ‘Safety of artificial intelligence-assisted optical diagnosis for leaving colorectal
polyps in situ during colonoscopy (PRACTICE): a non-inferiority, randomised controlled trial’, Lancet Gastroenterol. Hepatol., 2025.
C. Biffi et al., ‘A novel AI device for real-time optical characterization of colorectal polyps’, NPJ Digit. Med., vol. 5, no. 1, p. 84, June 2022, doi: 10.1038/s41746-022-00633-6.
C. Hassan, G. Balsamo, R. Lorenzetti, A. Zullo, and G. Antonelli, ‘Artificial Intelligence Allows Leaving-In-Situ Colorectal Polyps’, Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol.
Assoc., vol. 20, no. 11, pp. 2505-2513.e4, Nov. 2022,
doi: 10.1016/j.cgh.2022.04.045.
Repici A, et al. 2020 ‘Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial.’ Gastroenterology.159(2):512-520.e7. doi: 10.1053/j.gastro.2020.04.062.
Globally approved. Widely adopted.
GI Genius™ is CE-marked and FDA-approved. It is also approved in several other markets, including Australia, Canada, Switzerland, Singapore, Israel, the UAE, and the U.K., enabling widespread access to cutting-edge AI support in endoscopy
Publications & scientific evidence
No results found.